Forsøgsdyrenes Værn's research grants help animals
Forsøgsdyrenes Værn helps laboratory animals by supporting 4 exciting research projects which can replace experiments on animals. Your contribution makes it possible to continue funding reliable, modern technology which can pave the way for humane, animal-free research!
1)Animal-free test for toxicity of chemicals

The goal of this project is to present a method that replaces the use of animals to test chemicals inhalation toxicity to humans.
Proper labeling of chemicals relies on documentation of health effects following inhalation. Today this is done by exposing mice or rats for the chemical for up to 4 hours, while they are fixated and the animals that survive are observed for 14 days following the inhalation.
This test follows the ‘OECD Technical Guideline 436’ and the test causes the animals distress. An alternative method is therefore needed for assessing acute inhalation toxicity, without the use of animals. We at the National Research Centre for the Working Environment (NRCWE), have long worked with inhalation toxicity of impregnation spray products, and this work has shown that a modification of an existing method is very good at predicting inhalation toxicity.
The specific purpose of the present project is to do the necessary studies required as evidence that the method is usable, reliable, relevant and robust.
The mechanism of lung damage
Every year there are people who become sick after inhaling impregnation sprays developed for surfaces such as clothes, shoes, car windows, tiles and flooring. After a few hours they experience problems breathing. We have been working to uncover what happens in the lungs, and why some chemicals cause acute lung damage, while others don’t.
It turns out that the chemicals that cause acute lung damage affect a thin liquid film that covers the respiratory parts of the lungs. This liquid film is made up of lung surfactant. Lung surfactant is vital for lung function. If the surface tension when the lungs are compressed (this happens during each exhalation). If the surface tension does not fall, the alveoli will start to collaps. The result is that something that normally requires minimal work – breathing – suddenly becomes hard work, for experimental animals this can become fatal.
Reliable alternative method
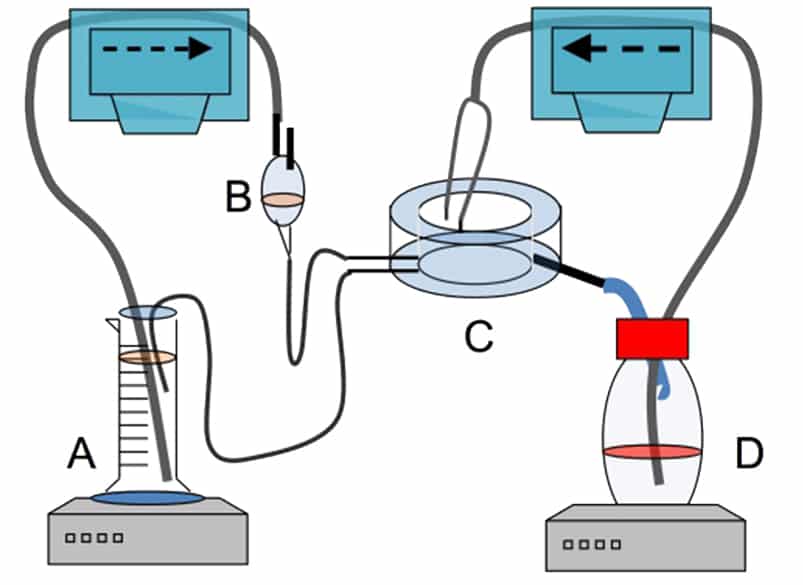
We use the method Constrained Drop Surfactometer (CDS). It is a commercialized instrument that does not involce the use of laboratory animals. Instead, surfactant from pigs or calves is removed from the lungs of already slaughter(ed)? animals.
We have rebuilt the CDS instrument so that it can simulate what happens in your lungs when you breathe air with chemicals e.g. from spray products.
The result of our studies clearly demonstrate that spray products are toxic to inhale both for humans and mice, also inhibit the lung surfactant function in the rebuilt CDS method. Likewise, products that do not inhibit surfactant function, is non-toxic to mice. In addition, we have shown that the method is suitable for testing pharmaceutical excipients and nanoparticles. The rebuilt CDS method has great application potential.
Documentation of the method
Although we have shown that there is concordance between the results from the CDS method and real life situations, there is still a lot work needed before the method can be accepted by the regulatory authorities. It is this work, this project will focus on.
2) Skin cancer model without use of animals
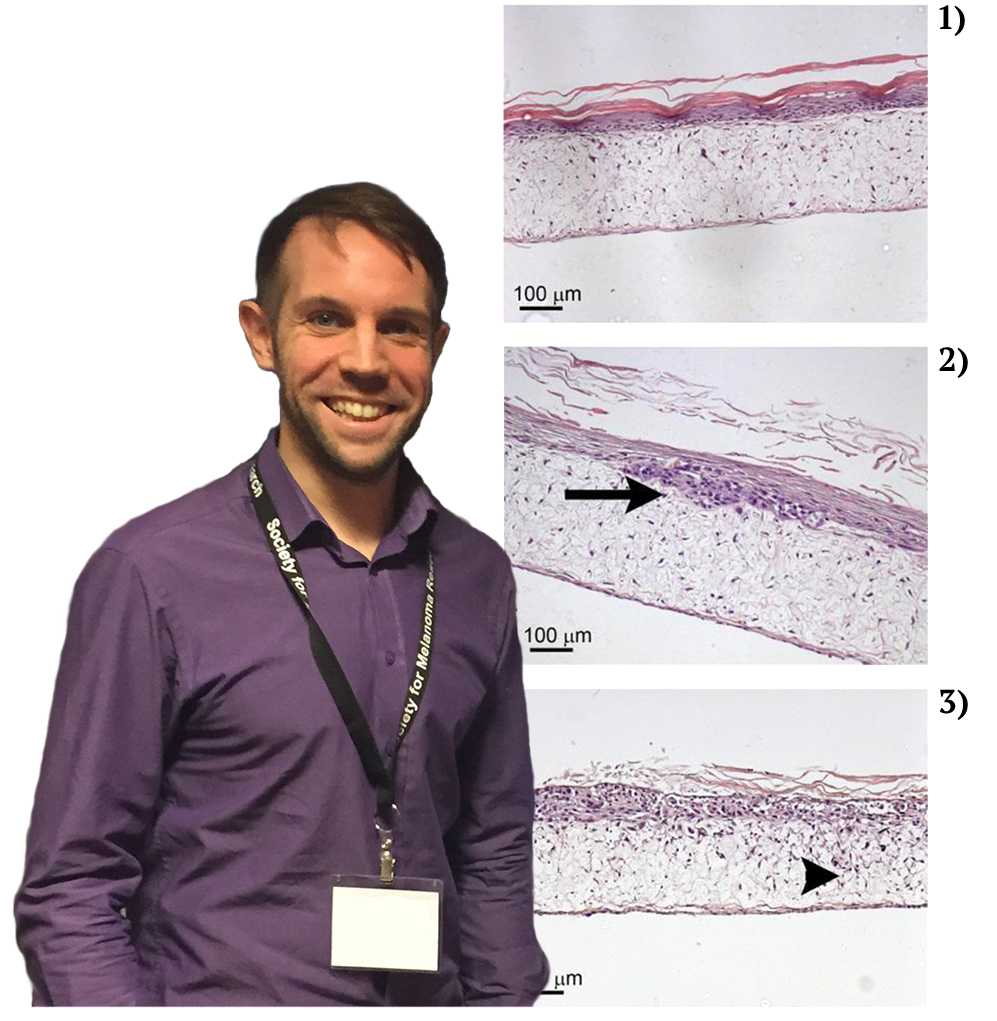
Malignant melanoma is the most deadly form of skin cancer, for which there are no consistently beneficial treatments. New therapeutic approaches for the treatment of melanoma focus on reducing tumour cell division and/or invasion, to slow tumour growth and prevent spread of the disease. Testing the safety and efficacy of new therapeutic strategies currently relies heavily on animal models, which may cause suffering and distress to the animals involved. Furthermore, mouse models aimed at studying the initial stages of melanoma development and invasion within mouse skin may not accurately represent human disease due to differences in the structure of skin between mice and humans. Therefore, to replace the use of mice for the investigation of local, early stage melanoma development, invasion and therapy response, I have recently developed and optimised an in vitro full-thickness melanoma skin equivalent model.
This novel assay accurately recreates the pathological progression of melanoma cells within their original human skin microenvironment as they divide and invade through the basement membrane, resulting in the breakdown of Type VII collagen. The aims of the current project are to modify the current protocol for constructing melanoma skin equivalents to eliminate the use for animal derived components such as bovine pituitary extract; to develop a nucleotide aptamer alternative to animal-derived antibody detection of Type VII collagen; and use the developed aptamer to validate the melanoma skin equivalent constructed with animal component free media by comparing breakdown of Type VII collagen with that observed in melanoma skin equivalents from a previous project created with media containing animal components. The current skin equivalent model is currently used within our lab as well as other labs for a wide range of applications; therefore adaptation of this model to an animal-component free methodology will ensure future studies using this skin equivalent are truly animal free.
3) Alternative to creation of antibodies in animals

In research and the field of life sciences it is a challenge to purify and isolate specific proteins efficiently. All cells produce an overwhelming number of different proteins. Therefore, it can be compared to finding a needle in a hay stack. Though this task can be lightened, if the proper tools are available. In the case of the needle and the haystack a magnet will do the trick. In much the same way some proteins ability to bind other proteins can be utilized, this is called protein-protein interaction. There are limitations to the physical properties and ethical concerns to the methods used today though. It is the aim of two students from the University of Southern Denmark to solve this. Anders Christian Hansen, who studies a master in biomedicine, and Mette-Marie Wendelboe Nielsen, who studies to be a civil engineer in chemistry.
The project was started in 2015 as part of a larger group of students, who participated in the international competition iGEM. Where students compete in the field of synthetic biology. The competition was originally started by Massachusetts Institute of Technology, MIT. The aim of the project was to replace the use of antibodies with peptide aptamers. Since it has been shown, that they have comparable abilities to create protein-protein interaction. After the competition is was decided that the project should be continued due to the importance.
The troubles with producing antibodies are, that it is tedious, expensive and not least, that they are produced in animals often mice, rats and rabbits. If an antibody against a particular protein, also known as a target protein, is wanted, the immune system of the animal is utilized. The immune system works by creating antibodies, when the organism is infected by a foreign body. Thereby restoring the health of the animal. In antibody production this is utilized by injecting the target protein into the animal. Thereby activating the immune system and the formation of antibodies. These are extracted by removing blood and/or the spleen of the animal. Where after they undergo several procedures, before the finished product is ready.
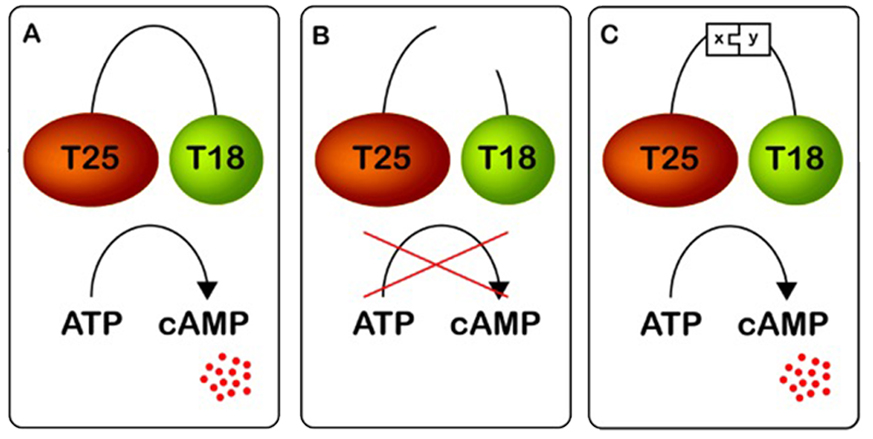
Our alternative is comprised of recombinant proteins, that can be produced in bacteria. When we have a target protein the first step is to find a peptide aptamer, that is able to participate in protein-protein interaction with the target. This is achieved by placing a DNA library, that consists of random DNA sequences, into a so called scaffold, which is an especially stabile protein. These two parts represents the peptide aptamer. By inserting both target and peptide aptamers in a reporter system, protein-protein interaction can be detected by a colour response, this is called a screening. This will allow us to sort the coloured bacteria, which contain potential peptide aptamers.
This system has been known for several years, though there are problems with the functionality. This occurs due to the generation of false positives, when the DNA library binds to another part of the system, rather than the target. This also results in a colour respons. We believe, that we have solved this problem in a way the will allow for an efficient screening. Which will lighten the work of determining the best suited peptide aptamer significantly. Now all that is left is to move the DNA sequence of the peptide aptamere to a production system in another bacteria and produce the wanted quantities.
In this way we are bettering the conditions for researchers by creating a cheap, efficient and high quality alternative to antibodies. At the same time we allow for better education of the researchers of tomorrow, since teaching experiments will be far cheaper. This together with the fact, that no animals will be killed during the manufacturing, leave us convinced, that our project will be paramount in the reduction of the use of laboratory animals.
4) Experiments using placental material shed light on transport of dangerous chemicals

In the field of reproductive toxicology the use of animal experiments have very poor predictive value. The length of pregnancy, the developing stages of the fetus are very different between animal species, and the placenta exhibits a larger degree of species difference than any other organ making it difficult to interpret animal data with respect to predicting fetal exposure in human pregnancy.
To investigate the human fetal exposure we use the human placental perfusion model. The ex vivo human placental perfusion model is a technically challenging and sophisticated model, which is an alternative to animal studies with the potential to reduce and replace animal tests on fetal exposure. Human placentas are donated directly after birth and taken to the laboratory to study the fetal exposure to environmental chemicals and endogenous substances. Perfusion models are very useful in studying effects and actions of various live tissues, in order to extrapolate the findings to a real life exposure situation. Placental perfusion models are most commonly used to investigate the exchange of substances between mother and fetus including potential metabolism of these, but also to study the effect of different factors on the placental tissue, including morbidity and hormone production alterations. We are developing the model to investigate the effect of different substances on the placental function, which can have significant impact on the well-being of the mother and the fetus during and after pregnancy.
The human placental perfusion facility in our laboratory in Copenhagen is close to the maternity ward at Copenhagen University Hospital (Rigshospitalet) from where we obtain viable placentas, and we have had ethics approval and logistics in place since 2004. In the placental perfusion model a single unit (cotyledon) from a human term placenta is re-perfused directly after birth. Fetal and maternal circulations are re-established, and the transport of the chosen test-substance can be investigated by adding the substance to the maternal compartment and follow the distribution of the substance over time into the placenta and across to the fetal compartment1. Inter-laboratory comparisons with two laboratories in Finland have validated the system2. Cellular transport mechanisms and pathways across the human placenta can be investigated after the experiment by confocal and electron microscopy of perfused placental tissue3,4. Data from our studies of human placental transport distinguish substance transport by molecular weight, size and degree of chlorination/bromination by in vivo, ex vivo and in vitro studies5-11. Our studies confirmed the importance of studying placental transport in an intact placenta, as the results could not be re-created using an in vitro cell model, and receptor-binding studies12.