Forsøgsdyrenes Værn held on 29th October 2019 a seminar entitled
New Visions in Science
Five speakers made some very exciting contributions – read below.
Alternatives and the Law
Rosemary Goddard Svendsen
She began by sketching the requirements the EU sets for
1) the implementation of animal-free methods,
2) the protection of laboratory animals,
3) the Animal Experimentation Council (Rådet for Dyreforsøg), for the industrial businesses and the institutions where experimentation is carried out and
4) for the individual researcher.
The government must support the development and promotion of alternative methods. The Animal Experimentation Council (Rådet for Dyreforsøg) must demand an explanation from an applicant as to how the person intends to implement the requirement to use animal-free methods in his or her planned reseach. Research establishments must help their staff to fulfil the law’s demands on animal-free methods – including ensuring that information on new knowledge is passed on to researchers and technicians.
Rosemary G. Svendsen estabished that the individual researcher is obliged to plan his or her research, so that it is carried as gently as possible and that animal-free methods are used whenever possible. In her talk she pointed out a number of differences between the Directive and Danish law – including that the Danish definition of the 3Rs (Reduction, Refinement, Replacement) is narrower than the concepts used in the Directive. The Directive’s very specific requirement that an evaluation of each research project must be undertaken, and that the result of the evaluation must be positive before permission can be given to carry out the research was not written into Danish law. That means that the evaluation of projects carried out by the Animal Experimentation Council (Rådet for Dyreforsøg) is less transparent and that there is a risk that the Danish evaluation does not include all the points listed in the Directive. The evaluation of an application must, for example, cover the planned use of animal-free methods. In answers given during question time, R.G. Svendsen explained, among other things, how the EU checks on whether the Directive has been correctly written into national law and what happens if errors and omissions are found.
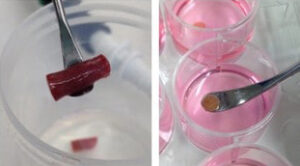
Human precision-cut tissue slices: a unique translational disease model
Rick Mutsaers, Ph.d., Aarhus Universitet
Machine Learning-based big data expression analysis - Closing the gap towards personalized medicine and pushing away from animal models
Ph. D. Kenneth Kastaniegaard, Biogenity
Broadly everything we do results in a data trail and the amount of data is growing so explosively that the world’s collective amount of data doubles every third year. This enormous amount of data is called Big Data and implies new possibilities for, among other things, analysis. There is a huge need for Big Data analysis in the medicines industry.
Biogenity’s vision is to optimise drug development through big data. The desire is to improve hypothesis testing through existing data and to increase transparency between diseases and animal models and in that way create the basis for eliminating certain animal experiments.
When a new medicine is developed, Big Data analysis of gene and protein expression is very valuable, as it can increase the success rate and thereby save companies millions of kroner in drug research and related animal experiments.
Traditionally a Big Data analysis in these types of data requires a biostatistician to carry out the manual and time-consuming work. Therefore, this type of analysis lies in a price class which only large pharmaceutical businesses can afford.
Biogenity’s technlogy is based on artificial intelligence and reduces the manual work i Big Data analysis with use of machine learning. The technology will thus reduce time and costs and make Big Data analysis available to small and medium sized businesses.
Animal derived antibodies: Time for change
Dr Alison Gray, Afabillity, Nottingham University
European expert group says no to antibodies developed in animals
Dr Gray stated that EURL-ECVAM* would soon come with a report and recommendations on animal-free antibody production. The report is being drafted by a scientific expert group, of which Dr Gray was a member. At the same time, the USA has set up a work programme on antibodies, and there is contact between EURL-ECVAM and the American programme, as international cooperation is particularly important in solving the problems. Dr Gray explained that it would be necessary to establish expert training and advisory options to help businesses and institutions to go over to use and production of animal-free antibodies, and that guidance from the EU could help animal experimentation authorities to deal with applications for permission to develop antibodies in animals.
* The European Reference Laboratory for Alternatives to Animal Experiments
Sequence defined non-animal-derived antibodies
Professor Stefan Dübel, Technische Universität Braunschweig
Professor Dübel stated in his talk that today there are three types of antibody on the market: a) polyclonale antibodies, which are generated in the blood of animals and are cheap to produce within a few weeks, b) monoclonal antibodies, which are typically developed in the spleen of an animal, are three times more expensive and take several months to produce. Polyclonal antibodies always have a mixed content and monoclonal antibodies, which it is intended should aim at a specific target often bind to something quite different. Both types are undefined and are not possible to reproduce exactly, in contrast with c) recombinant antibodies. Recombinant antibodies have the highest quality and have many advantages. They can be developed without use of animals – either from human blood or from synthetic genes. As the antibody’s sequence is defined and known, it can always be produced again in its original form. Recombinant antibodies are therefore always fully reproducible. They can be produced in the course of a few weeks and are fully reproducible so they bind more precisely to the target.
Forward-looking foreign firms use animal-free antibodies
Animal-free recombinant antibodies developed from phage display systems are the most used atibody type in products which require the highest antibody quality, namely therapeutic products for treatment of diseases. Large companies like Eli Lilly, GSK, Novartis, Astra Zeneca, Roche, Janssen and Merck use animal-free recombinant antibodies in therapeutic products for treatment of cancer, lupus, anthrax, asthma, colitis ulcerosa and many more. Animal-fee recombinant antibodies are also used in diagnostic testing equipment, but it is something that is just not advertised on the packaging.
Professor Dübel added that availability of animal-free recombinant antibodies was for long time limited for the ordinary researcher , but today they are sold by several commercial companies, for example, the big antibody producer Abcam and others such as Absolute Antibody, Abcalis and AdipoGen, who all advertise that they sell animal-free antibodies.
Conservatism and misunderstandings behind the use of animals
Animal-free antibodies give significant scientific advantages
Professor Dübel ended his talk by presenting the conclusions of a report on antibodies, which had been drafted during the year by EURL-ECVAM’s scientific advisory committee (ESAC), of which Professor Dübel himself us a member (as is Dr Alison Gray). The committee is writing in the report that the technology behind animal-free recombinant antibodies is well-established and well documented, that animal-free antibodies offer significant, extra scientific advantages, and that animal-free antibodies can replace conventional antibodies for almost all purposes. Finally, the scientific advisory committee concluded that animal-free recombinant antibodies will improve reproducibility of research and will have a positive influence on society. At the time of writing the report is expected to be published very soon.









